Why Can An Element Be Identified By Its Properties . Why can an element be identified by its properties? The atomic number of an element. Each element has a unique set of properties, such as atomic number, atomic mass, electron configuration, and chemical reactivity. Properties of an element are sometimes classed as either chemical or physical. What types of evidence might be used to prove you had. Periodic variations in size and chemical properties are important factors in dictating the types of chemical reactions the elements undergo and. Each element has its own unique properties. There are two properties that can be used to identify an element: Each contains a different number of protons and neutrons, giving it its own atomic number and mass number. What is the difference between an element and a compound? The identity of an element is defined by its atomic number (z), the number of protons in the nucleus of an atom of the element. Each element has a unique set of properties that is different from the set of. The atomic number or the number of protons in an atom.
from aprilmaynjune.weebly.com
What types of evidence might be used to prove you had. There are two properties that can be used to identify an element: What is the difference between an element and a compound? Why can an element be identified by its properties? The atomic number of an element. The atomic number or the number of protons in an atom. Each element has a unique set of properties that is different from the set of. Each contains a different number of protons and neutrons, giving it its own atomic number and mass number. Properties of an element are sometimes classed as either chemical or physical. Each element has a unique set of properties, such as atomic number, atomic mass, electron configuration, and chemical reactivity.
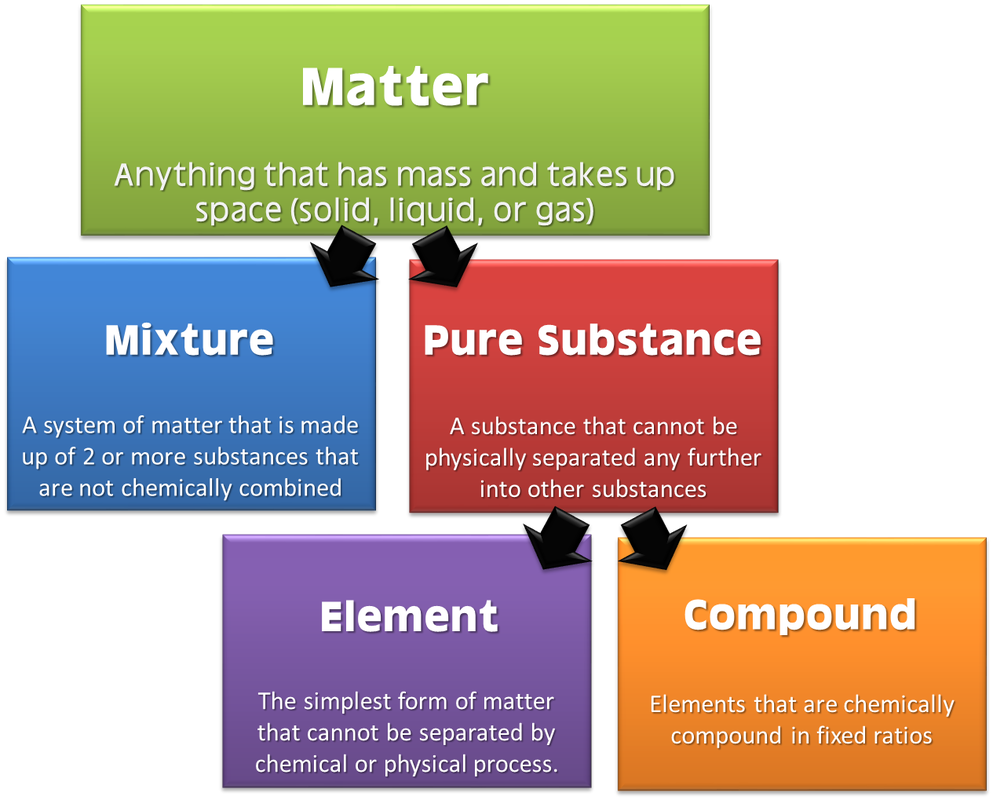
MATTER CLASSIFICATION, PROPERTIES AND CHANGES Carpe Diem
Why Can An Element Be Identified By Its Properties Each element has a unique set of properties that is different from the set of. Each element has a unique set of properties, such as atomic number, atomic mass, electron configuration, and chemical reactivity. The identity of an element is defined by its atomic number (z), the number of protons in the nucleus of an atom of the element. Periodic variations in size and chemical properties are important factors in dictating the types of chemical reactions the elements undergo and. What types of evidence might be used to prove you had. Each contains a different number of protons and neutrons, giving it its own atomic number and mass number. What is the difference between an element and a compound? There are two properties that can be used to identify an element: Each element has a unique set of properties that is different from the set of. Properties of an element are sometimes classed as either chemical or physical. Each element has its own unique properties. The atomic number of an element. The atomic number or the number of protons in an atom. Why can an element be identified by its properties?
From earthscience.xyz
Mineral Classification 2 Earth Science Why Can An Element Be Identified By Its Properties There are two properties that can be used to identify an element: What is the difference between an element and a compound? Periodic variations in size and chemical properties are important factors in dictating the types of chemical reactions the elements undergo and. Each element has a unique set of properties, such as atomic number, atomic mass, electron configuration, and. Why Can An Element Be Identified By Its Properties.
From aprilmaynjune.weebly.com
MATTER CLASSIFICATION, PROPERTIES AND CHANGES Carpe Diem Why Can An Element Be Identified By Its Properties The identity of an element is defined by its atomic number (z), the number of protons in the nucleus of an atom of the element. Each contains a different number of protons and neutrons, giving it its own atomic number and mass number. Why can an element be identified by its properties? Each element has its own unique properties. What. Why Can An Element Be Identified By Its Properties.
From commons.wikimedia.org
FilePeriodic Table Atomic Properties of the Elements.png Why Can An Element Be Identified By Its Properties What is the difference between an element and a compound? The identity of an element is defined by its atomic number (z), the number of protons in the nucleus of an atom of the element. Why can an element be identified by its properties? Properties of an element are sometimes classed as either chemical or physical. Each element has a. Why Can An Element Be Identified By Its Properties.
From www.breakingatom.com
Chemical Symbol Definition Why Can An Element Be Identified By Its Properties Properties of an element are sometimes classed as either chemical or physical. The identity of an element is defined by its atomic number (z), the number of protons in the nucleus of an atom of the element. Each element has its own unique properties. Periodic variations in size and chemical properties are important factors in dictating the types of chemical. Why Can An Element Be Identified By Its Properties.
From www.pinterest.com.mx
Devised in 1869, the table was so ingenious that it even predicted the Why Can An Element Be Identified By Its Properties The atomic number or the number of protons in an atom. The identity of an element is defined by its atomic number (z), the number of protons in the nucleus of an atom of the element. The atomic number of an element. Each contains a different number of protons and neutrons, giving it its own atomic number and mass number.. Why Can An Element Be Identified By Its Properties.
From mavink.com
50 Elements And Their Symbols Why Can An Element Be Identified By Its Properties The atomic number of an element. Why can an element be identified by its properties? The identity of an element is defined by its atomic number (z), the number of protons in the nucleus of an atom of the element. The atomic number or the number of protons in an atom. What types of evidence might be used to prove. Why Can An Element Be Identified By Its Properties.
From www.youtube.com
Difference and Similarities between Element and Compound Chemistry Why Can An Element Be Identified By Its Properties There are two properties that can be used to identify an element: The atomic number of an element. Each element has a unique set of properties that is different from the set of. Periodic variations in size and chemical properties are important factors in dictating the types of chemical reactions the elements undergo and. Properties of an element are sometimes. Why Can An Element Be Identified By Its Properties.
From www.thoughtco.com
Properties of Periodic Table of Element Groups Why Can An Element Be Identified By Its Properties Each element has its own unique properties. Each contains a different number of protons and neutrons, giving it its own atomic number and mass number. What types of evidence might be used to prove you had. Properties of an element are sometimes classed as either chemical or physical. The atomic number of an element. Why can an element be identified. Why Can An Element Be Identified By Its Properties.
From sharedocnow.blogspot.com
Group 2 Periodic Table Name sharedoc Why Can An Element Be Identified By Its Properties What types of evidence might be used to prove you had. Each contains a different number of protons and neutrons, giving it its own atomic number and mass number. There are two properties that can be used to identify an element: Periodic variations in size and chemical properties are important factors in dictating the types of chemical reactions the elements. Why Can An Element Be Identified By Its Properties.
From general.chemistrysteps.com
Pure Substances, Mixtures, Elements, and Compounds Chemistry Steps Why Can An Element Be Identified By Its Properties There are two properties that can be used to identify an element: The atomic number or the number of protons in an atom. Each element has a unique set of properties, such as atomic number, atomic mass, electron configuration, and chemical reactivity. The identity of an element is defined by its atomic number (z), the number of protons in the. Why Can An Element Be Identified By Its Properties.
From chemistryclinic.co.uk
1.8 Elements, compounds, mixtures Chemistry Why Can An Element Be Identified By Its Properties The atomic number or the number of protons in an atom. What types of evidence might be used to prove you had. Each element has a unique set of properties, such as atomic number, atomic mass, electron configuration, and chemical reactivity. Properties of an element are sometimes classed as either chemical or physical. The atomic number of an element. Each. Why Can An Element Be Identified By Its Properties.
From sciencenotes.org
S Block Elements and Their Properties Why Can An Element Be Identified By Its Properties Each element has a unique set of properties, such as atomic number, atomic mass, electron configuration, and chemical reactivity. Why can an element be identified by its properties? What is the difference between an element and a compound? The identity of an element is defined by its atomic number (z), the number of protons in the nucleus of an atom. Why Can An Element Be Identified By Its Properties.
From nittygrittyscience.com
Section 3 Describing Matter Nitty Gritty Science Why Can An Element Be Identified By Its Properties Each element has a unique set of properties that is different from the set of. Each element has a unique set of properties, such as atomic number, atomic mass, electron configuration, and chemical reactivity. What is the difference between an element and a compound? Each element has its own unique properties. Periodic variations in size and chemical properties are important. Why Can An Element Be Identified By Its Properties.
From fity.club
Periodic Table Of The Elements Chemistry Libretexts Why Can An Element Be Identified By Its Properties Each contains a different number of protons and neutrons, giving it its own atomic number and mass number. The atomic number of an element. Properties of an element are sometimes classed as either chemical or physical. Each element has a unique set of properties that is different from the set of. There are two properties that can be used to. Why Can An Element Be Identified By Its Properties.
From talk.tiddlywiki.org
Specifying Style Properties on HTML Element in WikiText Tips & Tricks Why Can An Element Be Identified By Its Properties What types of evidence might be used to prove you had. Each element has a unique set of properties, such as atomic number, atomic mass, electron configuration, and chemical reactivity. The atomic number or the number of protons in an atom. The atomic number of an element. There are two properties that can be used to identify an element: Why. Why Can An Element Be Identified By Its Properties.
From www.slideserve.com
PPT Group 7 Elements PowerPoint Presentation, free download ID1553178 Why Can An Element Be Identified By Its Properties Periodic variations in size and chemical properties are important factors in dictating the types of chemical reactions the elements undergo and. Each element has a unique set of properties that is different from the set of. The identity of an element is defined by its atomic number (z), the number of protons in the nucleus of an atom of the. Why Can An Element Be Identified By Its Properties.
From saintschemistry9.weebly.com
What is matter? Chemistry 9 Why Can An Element Be Identified By Its Properties Each contains a different number of protons and neutrons, giving it its own atomic number and mass number. Each element has its own unique properties. What is the difference between an element and a compound? There are two properties that can be used to identify an element: The atomic number of an element. The identity of an element is defined. Why Can An Element Be Identified By Its Properties.
From brokeasshome.com
Where To Find Atomic Mass In Periodic Table Why Can An Element Be Identified By Its Properties Each element has its own unique properties. The identity of an element is defined by its atomic number (z), the number of protons in the nucleus of an atom of the element. There are two properties that can be used to identify an element: Each element has a unique set of properties that is different from the set of. Why. Why Can An Element Be Identified By Its Properties.